COVID-CTPRED project
Patients cohort data (chest-CT images and CRF metadata) were collected in a regional COVID
reference University Hospital center (CHU Saint-Etienne, France).
Study inclusions criteria are:age ≥18 years, clinical suspicion with respiratory
signs of COVID19 requiring hospitalization, Chest-CT at ER admission, RT-PCR sampling, and written
informed consent.
Longitudinal data are collected from the emergency department (ER) admission time point
until patient discharge (follow-up duration :1 month). Note that the number of CT-scans and
metadata may vary depending on patient’s clinical course.
Live detailed statistics are available of the image database as well
as the nature of the imaging and corresponding metadata available.
Use and publication policies
The COVID-CTPRED clinical trial database contains pseudonymized data (images, clinical and
biological metadata)(“DATABASE”). The authorization to access and exploit the results
extracted and/or calculated from the DATABASE is conditional to the implicit agreement with
the following points:
1) Authorized users are not allowed to redistribute in any form the data from the
COVID-CTPRED database.
2) Citation rules when publishing any results obtained from the latter.
See the FAQ section here
Any inquiries should be directed to the sponsor representative (Pr P.Croisille, Chairman of
Radiology and Nuclear Medicine, CHU Saint-Etienne mail)
To ensure that patients' rights and the internal policy of CHU St-Etienne are respected,
this database is made available to the scientific community in a secure manner.
To do this, it is necessary for people wishing to participate in this
project to identify themselves to the CREATIS laboratory before being
able to access the public part of the database.
Two viewer services have been implemented as well.
Admission and subsequent CTs during hospitalization:
Naming convention: CHUSE_COVID_CTPRED-F1_Patient_F2_F3
F1: Protocol variant (S, SI, or SIV)
F2: 4-digits unique patient number
F3: 1-digit incremental CT index during follow-up (n=1 to 5)
Clinical report form (CRF) data are collected on a REDCAP internal server and exported in csv format.
Data includes: comprehensive personal and medical history, medications, admission vital
signs, pulmonary xray/CT/US results, admission biology / blood gas / PCR, patient initial
outcome, ICU hospitalization data (admission/mid- /end-stay (vital signs, biology, blood gas,
pulm.assistance parameters(@ followup time-points: J0,J4,J7,J14J21,J28)), chest CT findings,
outcome at J30.
As explained just above, there are three protocol variants.
Each patient may have one or more variants. This graph shows the distribution
of samples by type of variants.
All public samples are from SIV protocol variants and corresponds to a first exam.
Clinical data on these patients were randomised for confidentiality reasons.
The images below are examples of different acquisition for a sample subject that did a full exam.
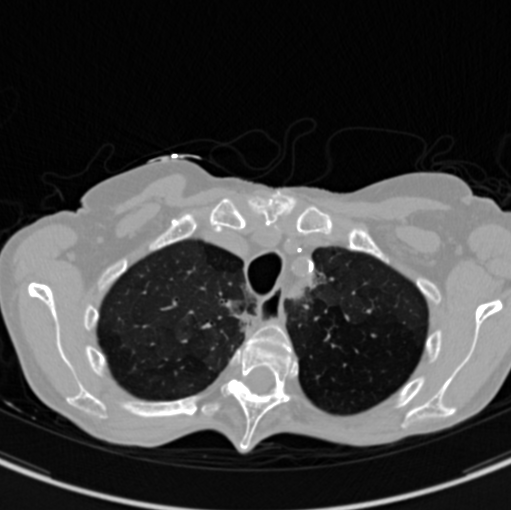
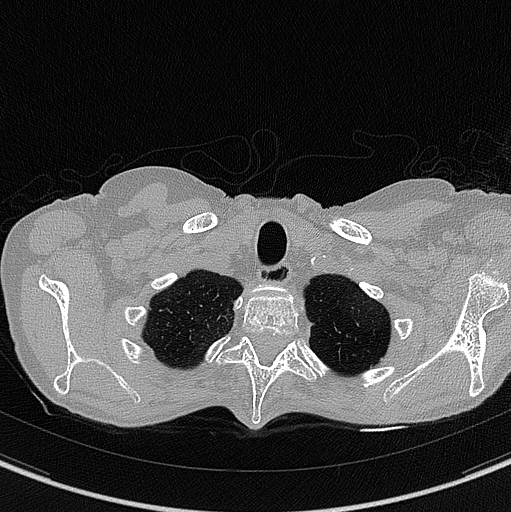
Inspiration acquisition without injection. Left mediastin reconstruction, rith parenchyme reconstruction.
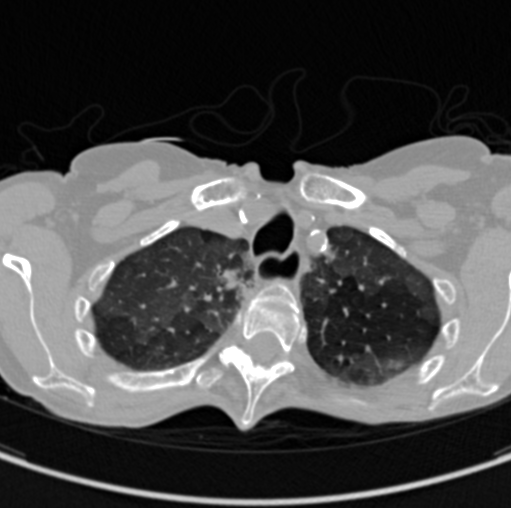
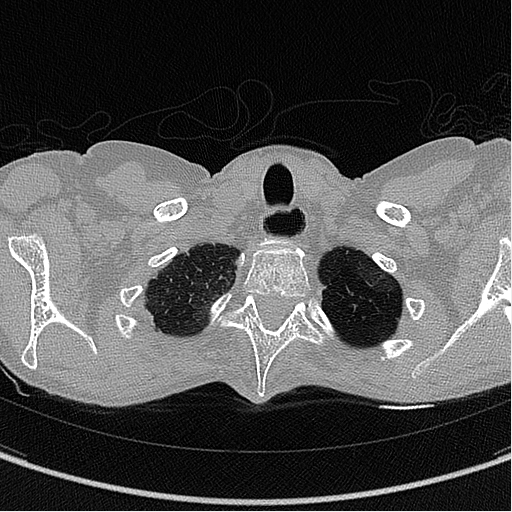
Expiration acquisition without injection. Left mediastin reconstruction, rith parenchyme reconstruction.